|
|
Post by ruth on Nov 4, 2009 13:15:40 GMT -5
i am only growing one species so far. i did not have growth after 24 hours, very small amount at 48 hours, but lots of channeling through the media. so......last night i put a group of oat flakes on one side of the culture dish. and.......this morning i have a tremendous amount of growth mine looks like a match for slime mold, i've seen the same in pics, i'll try to locate it. it is very hard for me to get pics that are clear, but you will be able to see. i've put up a few on my photo site. the spores are inbetween the masses of the clear/white mycelia 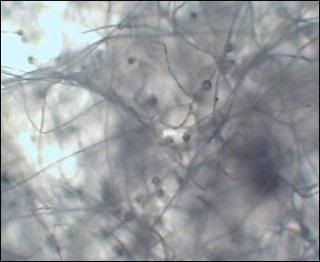 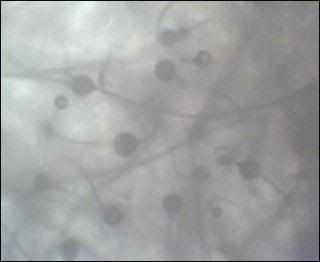 channeling 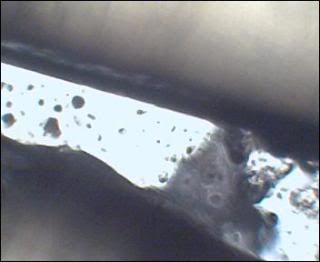 |
|
|
|
Post by ruth on Nov 4, 2009 13:33:50 GMT -5
tinyurl.com/yhjg6fadictyosteum The primary diet of D. discoideum consists of bacteria,dsc.discovery.com/news/2009/09/08/slime-mold-computer.htmlruns on slime mold instead of electricity It's probably the nastiest, slimiest computer in the world. Powered by oat flakes instead of electricity, scientists in the UK have developed a rudimentary computer using a slime mold they have affectionately named Plasmobot.
|
|
|
|
Post by dc10801 on Nov 4, 2009 13:50:21 GMT -5
probably came from the oat flakes then
|
|
|
|
Post by ruth on Nov 4, 2009 14:40:57 GMT -5
i'm not saying there was nothing growing in the petri dish before the oat flakes it was the channeling that led me to believe it needed another food source and all the literature i read said oat flakes or tree bark. there was sparse branched clear hyphae of the same that i photographed before adding the flakes to the culture dish. 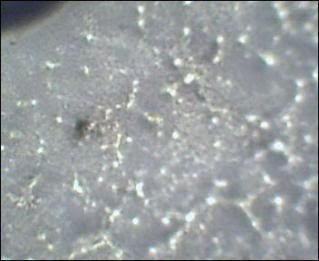 ![]() i192.photobucket.com/albums/z157/ruthlyons_2007/Picture053-2.jpg i192.photobucket.com/albums/z157/ruthlyons_2007/Picture053-2.jpg [/img] i worried about the oat flakes being contaminated. i got a box of instant flavored individual packs and picked the oats from the top hoping since they were processed, there would be less contaminants. i'm going to work now, i'll stop by some stores to find another prolab kit to get culturing and send it off for id. |
|
|
|
Post by Sidney on Nov 4, 2009 14:56:17 GMT -5
Ruth, this is just great. I'm so glad you're already seeing growth in the petri dish.
I do have a request though. Could you please move your post to the thread Toni started for me? DC, could you please do the same?
If we can get all the comments in one spot it will help all of us to keep track of our test results.
Jill just posted some great information to that thread too.
Many thanks to the three of you.
|
|
|
|
Post by kammy on Nov 4, 2009 15:17:12 GMT -5
Hi Ruth, great photos! These top two are exactly like the 'fungus/mold' that I have been seeing all along as the "Morgellons Fungus" in all human samples - so you think it is a slime mold? Now, if we can just get the lab to 'officially' name it, we will know the culprit at work inside of us. You might want to ask them if they specifically identify slime molds and are any of the other fungi/molds that people have reported within their tests in the slime mold families? I'm starting to wonder if this one isn't being overlooked by the labs because my recent test came back negative for fungus? It would be great for us all to be able to go to a doctor or mycologist and say... look specifically for this mold! Oh hallelujah, that would be progress! Great work, Ruth! |
|
|
|
Post by Acacian Immolation on Nov 4, 2009 19:29:18 GMT -5
nothing personal.. but why label the thread pro lab culture ...
when it wasn't done by lab professionals or in a lab?
i don't get it.
|
|
|
|
Post by Sidney on Nov 4, 2009 19:56:49 GMT -5
The name of the manufacturer is PRO-LABS. The medium used is designated to culture MOLD.
I don't know what sort of medium Kammy and Jeany have been using, but they too have grown mold and one of them mentioned they can grow it in a matter of hours.
Does this help?
|
|
|
|
Post by kammy on Nov 4, 2009 20:05:24 GMT -5
Yes, we understand their product name is "Pro Labs"... you send it off to them, I'm sure they have some sort of lab setup?
I'm thinking Morgs will grow in most any kind of medium... any agar, probably anything that's wet?
I don't see 'fungal' growth within a couple of hours but I do see that the other components are starting to respond, it usually takes a couple of days to start actually seeing visible fungal growth.
|
|
|
|
Post by dc10801 on Nov 4, 2009 20:24:18 GMT -5
That was my only concern I know I don't have a lot of patience at this point, but adding anything outside of what comes off us kinda lessens the credibilty of the results...I know its probably as agonizing as watching paint dry.
Some blood cultures actually take weeks to show some fungi.
Grains oats cereals etc harbor there own fungi, I wouldn't want to give anybody ammo for a rebuttle of what I grew thats all I meant.
You know..I had to proofread this post like 6 friggin times...brain fog had me typing like an idiot...I hope you get my point, I wasn't trying to negatate anyones efforts. I should stop now lol
|
|
|
|
Post by dc10801 on Nov 4, 2009 20:26:38 GMT -5
grrr still a typo...what the heck is "negatate"!!!!!!!!!!! I meant negate
|
|
|
|
Post by kammy on Nov 4, 2009 20:44:12 GMT -5
Well... I think Ruth HAS captured the Morgellons "Fungus" whether it be in her original specimen or in the oats. That's easily determined by her next test, which she is preparing. If this same fungus/mold is present without the addition of the oats in the next dish, then we will know the true source was there to begin with, if it is not - she will add one more suspect food to the list and determine whether to look closer at oats?
It seems to me that it was there to begin with, the oats would have needed more than a day to show this kind of growth and I believe she said, this photographed mold growth was on the other side of the Petri Dish from where the oats were? Still, I agree, the less variables in the dish the better but still an excellent experiment!
|
|
|
|
Post by Sidney on Nov 4, 2009 22:46:42 GMT -5
Yes, we understand their product name is "Pro Labs"... you send it off to them, I'm sure they have some sort of lab setup? I'm thinking Morgs will grow in most any kind of medium... any agar, probably anything that's wet? I don't see 'fungal' growth within a couple of hours but I do see that the other components are starting to respond, it usually takes a couple of days to start actually seeing visible fungal growth. Sorry, I thought I had read you were able to see growth within only a few hours. They would certainly have a lab if they're going to sell the kits and then charge a fee for identifying mold growth. What I fail to understand is if "we" are able to grow mold using specimens from our own body, why haven't any of the scientists involved grown anything? I'm assuming they must surely have tried. My understanding has always been that specific culture mediums must be used in order to identify any specific mold or fungus. My impression has always been that different species of mold-fungus require different growing mediums. I am encouraged by what I've been able to grow ONLY because the growing medium in the Pro-Labs culture kits are targeted for MOLD.....but again, there are many species of mold. Toni-Sue brought up a good point tonight. What about one little drop of urine, one drop of blood added to the culture? We can only wait and see and hope for some results that will lead us somewhere. |
|
|
|
Post by ANTHILL on Nov 4, 2009 22:48:21 GMT -5
grrr still a typo...what the heck is "negatate"!!!!!!!!!!! I meant negate I thought it might be intresting to see if it was a word and guess what it is  Negatate Negatate Definition: v. tr., To clean up another person's mess, only to have all their garbage dumped right on top of you. n., A person who tries to help other people solve their problems but always ends up in deep doo-doo. Pronunciation: neh-guh-tayt Sentence: Jonny became the negatate when Mittens attacked him for trying to clean out his litterbox Etymology: From negated, meaning to be cancelled out by another action |
|
|
|
Post by Sidney on Nov 4, 2009 23:03:13 GMT -5
Good one, Ant.
On a different note:
GO, YANKEES!!!! Yankees 7 Phillies 3, 7th inning.
LOVIN' it. Ye HAW!!!
|
|
|
|
Post by ruth on Nov 5, 2009 0:17:30 GMT -5
i found another culture kit today. when i look at culturing the slime mold, it is difficult without a food source.i know from experience it needs a food source, so, i believe if i can sterilize the flakes i use would that be right? the growth i had migrated towards the flakes.. it is all over the plate now, so i will not put anything in with this next plate. the growth will be slow. i do not know if it can form without food. it may stay liquid?  ?
this article doesn't include sterile anythingas with water, etc. a certain amount of growth will take place. maybe i should just send the minimal amount of growth to the identifying lab? what if it reverts to liquid form since there is no food for other formations? by the way.......the pieces of flakes have all been engulfed.........the growth is horrific  miriam-english.org/files/slime-molds/AmateurScientist_slime-mold.html miriam-english.org/files/slime-molds/AmateurScientist_slime-mold.html"Place a small fragment of selerotium on the platform and wet it with a drop of water. Within a few hours the organism will awaken from its deathlike torpor. Keep the unused portion of sclerotium in the refrigerator. When the plasmodium has emerged and has begun to seek food, put a flake of uncooked oats in contact with the rapidly spreading growth. Use old-fashioned rolled oats, not the 'quick' variety. "Keep the covered dish at room temperature in an area that does not get direct sunlight. As the plasmodium increases in size, place more oat flakes along the growth front. A rapidly moving plasmodium will consume a larger number of oat flakes than a sluggish one. In either case the feeding organism will show a decided preference for fresh oat flakes and will abandon partially digested ones. Flakes that have first been moistened with a drop of water are accepted more readily than dry oats. To maintain a clean culture, transfer the organisms to a fresh sheet of filter paper or paper toweling weekly, avoid overfeeding and remove abandoned food that shows signs of becoming moldy or slimy. Occupied oat flakes (those covered with yellow plasmodium) or sections of paper bearing plasmodium can he transferred repeatedly to clean sheets of paper to perpetuate the plasmodial stage. |
|
|
|
Post by bannanny on Nov 5, 2009 1:05:50 GMT -5
Here's the pic from your link above ruth. I saved it cuz it looks just like the stuff I get out of my hands. The minute I get out of bed in the morning I go right outside to rub them out cuz they're so sticky from the gel oozing out. I get rolls of these things to come out every time. Saw a good sized one in my poop today too. The pic also looks like some of the things I've been finding outside, right along side the barely visible web-like strands.  I think slime mold is the right track to be on guys... don't think it's a natural one tho. More in the mutated altered category. hugs ~~ bannanny |
|
|
|
Post by kammy on Nov 5, 2009 1:11:58 GMT -5
i found another culture kit today. when i look at culturing the slime mold, it is difficult without a food source.i know from experience it needs a food source, so, i believe if i can sterilize the flakes i use would that be right? the growth i had migrated towards the flakes.. it is all over the plate now, so i will not put anything in with this next plate. the growth will be slow. i do not know if it can form without food. it may stay liquid?  Let me see if I can help you? Let me clear up what I think I've read - The way this kit operates is you add water to a powder that forms the Petri Dish gel or agar and you place this semi-liquid gel in the Dish and let it sit up for a couple of hours before you place your specimens in it? This fungus/mold you've shown above will grow in most anything, all it needs is a little moisture. It looks like D.D. a whole lot, we talked about it recently, but it only matches one of the stock photos in the upper right hand corner in its growth characteristics, none of the other characteristics are being seen. And, I don't believe anyone has come back from a lab test so far with D.D. in their dish? BUT... it could be D.D., we're obviously dealing with some sort of lab creation, it could be a mutated version of it? ProLabs needs to be asked if they test for D.D. or the slime molds, so that if it comes back as not there, you will know that they tested for it? You want as few variables as possible in the dish, you have 3 things that could be growing our suspect mold. 1 is the water you're mixing to make the gel agar, 2 is the sample itself, and 3 is the oats. Use as pure water as you can find when mixing your agar solution. You say, "it may stay liquid"? What may stay liquid? The agar has nutrients in it that will allow the fungus/mold to come to life, it just may not be as fast as with added 'food'. When you look into the fungus in the dish with your microscope, are you seeing any spheres yet? I noticed that Frito just purchased a couple of dishes and asked over on Sid's thread, what you gals are putting in your dishes as specimens so she can do a similar test? Also, these Morg particles love heat... put your dish in a closed box and turn the dish upside down - if your agar is not runny... this will speed up growth by creating a greenhouse environment. And, be careful when you open the dishes to view them with your microscopes, make sure all fans off in the room. Just trying to help... |
|
|
|
Post by ruth on Nov 5, 2009 13:03:31 GMT -5
in the prolab kit, the the media is liquid. it needs one hour to set. the problem i have is many of us have had
the doctor order cultures and without results.my contention here is that the proper food source for M is not included in all culture medias. and that M can easily revert to a liquid form without the proper food for it to do its cycle. as sid said.......these are specific for molds....i will have faith and not put any other food source into the new petri dish............plus i am making sure i have the red, blue, green, brown, black colored fibers put in the dish there is thick white cottony growth covering about 2/3 area of the 1st dish. it makes me sick to see it grow so abundant. it is what i have seen for years with only sparse representation until now. good Lord   the surprising thing is one of the oat flakes has been moved about an inch from where it started and is becoming amber goo. i can hardly wait to see what else is surprising |
|
|
|
Post by ruth on Nov 5, 2009 13:21:36 GMT -5
bananny,
the pic you posted........go down to:
Wandering myxamoebae
that reminds me of the pre oatflake culture pic
|
|